Swansea volunteers first to come forward for Covid vaccine trial
14 May
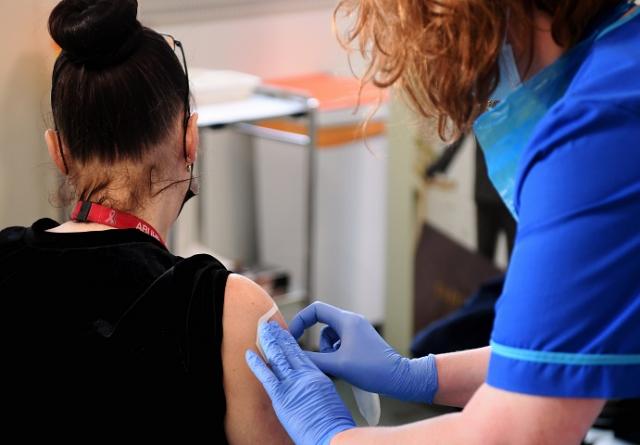
Volunteers from Swansea were the first in Europe to come forward to take part in an international clinical trial of a new COVID-19 vaccine.
The Medicago vaccine study is running across 14 sites in the UK, including Public Health Wales, which has now finished recruiting in Swansea.
It’s the first study to test the effectiveness and safety of the plant-based Coronavirus-Like Particle Vaccine (CoVLP).
The vaccine has already been through early phase human studies and now requires testing on a large scale. The study involves 1,500 people across the UK.
Last month, Public Health Wales began recruiting volunteers living in Swansea and surrounding areas.
The collaborative team also includes Health and Care Research Wales, Swansea Bay University Health Board and Swansea University Medical School.
Dr Brendan Healy is Principal Investigator for the Medicago trial and Consultant in Microbiology and Infectious Diseases at Public Health Wales.
He said: “We are very proud that Public Health Wales was the first site in the UK and Europe to enrol individuals in Swansea into this.
“I am very grateful to staff across Health and Care Research Wales, Swansea Bay University Health Board and Swansea University that worked so hard to achieve this.
“As a group we are extremely grateful to all the individuals that came forward to help with the trial, which is investigating vaccine produced using novel plant-based technology.
“It will generate valuable data that will help the global battle against COVID-19 over the long term. One of the advantages of this technology is that it enables vaccine to be produced at scale.”
The vaccine is produced in a form of coronavirus-like particles, known as CoVLPs, which are about the same shape and size and look very similar to live coronaviruses.
However, they do not have any viral genetic material which means they cannot cause the disease.
The CoVLPs are combined with an adjuvant (an ingredient that may enhance the body’s immune response) before the vaccine is given.
This allows for a smaller dose of the vaccine to be given, meaning more doses would be available to vaccinate more people, once the vaccine is approved.
As well as the 14 sites in the UK, the Medicago vaccine study is taking place in multiple sites in the United States, Canada, Europe and Latin America. It will enrol up to 30,000 volunteers worldwide.
Dr Healy said the beneficial effects of the existing Covid-19 vaccines were now being seen.
But, he added, it was important more were developed to give greater choice and the ability to select vaccines based on their individual benefits.
“Going forward, we will need a variety of options and this is a great opportunity to help us evaluate another vaccine against Covid-19,” he said.
Health and Care Research Wales is nationally coordinating research and study set-up in Wales. You can find out more by clicking here.