Wales leads pioneering gene therapy trial shown to slow progression of Huntington’s disease
26 September
A Welsh research centre, funded by Health and Care Research Wales, is at the forefront of a major breakthrough in the treatment of Huntington’s disease after playing a leading role in a global gene therapy trial.
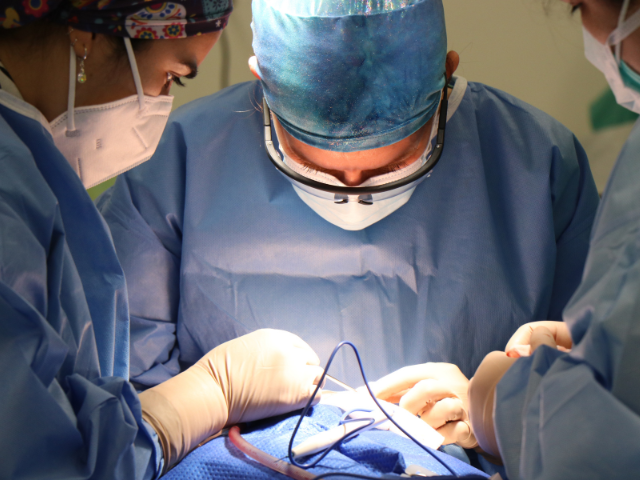
The Advanced Neurotherapies Centre (ANTC), based at Cardiff University, is the only UK surgical site to have participated in the trial, which tested a gene therapy known as AMT-130 in people with Huntington’s disease.
The trial has shown that patients receiving the therapy experienced a 75% reduction in disease progression compared to a matched cohort not receiving the treatment, marking the first time a drug trial has demonstrated a sustained, statistically significant slowing of Huntington’s disease progression.
Huntington’s disease runs through families, killing brain cells, and resembles a combination of dementia, Parkinson's and motor neurone disease. The first symptoms tend to appear in your 30s or 40s and is normally fatal within two decades – opening the possibility that earlier treatment could prevent symptoms from ever emerging.
AMT-130 permanently introduces new functional DNA into a person’s cells. It consists of particles of a harmless, empty virus, plus a set of instructions encoded in custom-made DNA. The virus is injected directly into a part of the brain called the striatum which is particularly vulnerable in Huntington’s disease. This is done using a highly complex neurosurgical technique called stereotactic surgery, in which tiny tubes called catheters are guided to the right part of the brain, supported by live MRI images. Once in the brain, the virus particles enter the neurons and release the DNA cargo.
Professor William Gray, Director of the Advanced Neurotherapies Centre and Senior Research Leader at Health and Care Research Wales, who performed the gene therapy surgeries said:
“This is a landmark result for patients and families affected by this devastating disease. Prof Anne Rosser and I are proud to have collaborated with Professors Tabrizi and Wild in London recruiting our and their patients to surgically deliver this innovative therapy directly into the brain in Cardiff.
Cardiff is the only UK site – and one of just a few globally – performing these gene therapy surgeries. We are deeply grateful to the patients who bravely volunteered for this experimental trial, to the sponsor uniQure, and to the exceptional clinical and research teams here in Cardiff who are helping turn these therapies into reality.”
Cabinet Secretary for Health and Social Care Jeremy Miles added:
Huntington’s disease is an absolutely devastating condition that runs through families, affecting movement, cognition and behaviour. For Welsh researchers to have contributed to this study by carrying out complex neurosurgeries in Cardiff - the only site in the UK with the capability to perform them – is a testament to how strongly Wales is able to support research into life-changing and even life-saving medicines for the benefit of people around the world.”
The trial sponsor, uniQure, plans to submit an application to the US Food and Drug Administration early next year for accelerated approval to market the therapy, with submissions in the UK and Europe to follow.
Keep up to date with the work of the Advanced Neurotherapies Centre by signing up to our newsletter.